The QM software for your laboratory
The QM software for your laboratory
Success Stories
Find out first-hand how laboratories use roXtra to make their laboratory quality management efficient, compliant and future-proof. Whether ISO 15189, GMP, GLP or comprehensive risk management - roXtra offers customized solutions for the specific requirements in laboratories.
Our blog provides you with valuable insights into accreditation and re-accreditation, the practical implementation of quality management in the laboratory as well as best practices and application examples from the industry.

Digital quality management at the laboratory service provider
TRIGA-S Scientific Solutions
Get advice now
Get to know roXtra in a non-binding and free online presentation.
What does quality management in the laboratory mean?
Quality management in the laboratory encompasses all measures, processes and systems that ensure that tests and analyses are carried out reliably, precisely and reproducibly. It is based on standards such as ISO 15189 for medical laboratories or ISO 17025 for testing and calibration laboratories and ensures that legal and regulatory requirements are met.
Why is QM important in the laboratory?
A well-implemented quality management system (QMS) improves the accuracy and reproducibility of laboratory results, minimizes sources of error and ensures efficient documentation. It contributes to the safety of patients and customers, optimizes work processes and increases the competitiveness of the laboratory. It also facilitates accreditations, audits and certifications, which strengthens the credibility and compliance of the laboratory.
In short, quality management in the laboratory is crucial to ensure high standards, reliability and efficiency - and thus to guarantee confidence in the results obtained.
What is the aim of a QM system according to ISO/IEC 17025?
ISO/IEC 17025 specifies the requirements for quality management systems in testing and calibration laboratories. Its central objective is to ensure technical competence and the reliability of results. A QM system in accordance with this standard is intended to ensure that laboratories apply validated procedures, take measurement uncertainties into account and maintain standard-compliant documentation.
The standard also promotes continuous improvement, transparency and compliance with legal and regulatory requirements. Structured quality management increases the trustworthiness of test and measurement results, which is essential for customers, authorities and accreditation bodies.
How do you record and analyze errors and deviations in the laboratory?
Errors and deviations in the laboratory must be systematically recorded, analyzed and corrected. QM software with action management facilitates this process by documenting deviations, analyzing causes and providing automatic workflows for corrective and preventive actions (CAPA).
Digital tools ensure transparent tracking, deadline control and complete documentation - essential for audits and certifications. Software-supported deviation management improves efficiency, compliance and quality assurance in the laboratory.
Which software or digital tools can improve quality management in the laboratory?
Modern software solutions facilitate quality management in the laboratory by automating processes, simplifying documentation and ensuring compliance with standards such as ISO 15189 or ISO/IEC 17025. Document management systems (DMS) support the versioning and approval of SOPs, while action management tools record deviations, analyze causes and control CAPA processes.
In addition, laboratory information and management systems (LIMS) improve the traceability of samples and results. Audit and training software helps to document evidence and meet certification requirements. The digitalization of quality management significantly increases efficiency, traceability and compliance in the laboratory.
One software, many application areas.
Efficient quality management
With roXtra Documents you can manage your QM documentation in the laboratory - from test procedures and service specifications to hygiene management and SOPs - effortlessly and in compliance with regulations. Our document management system (DMS) supports the entire document lifecycle in accordance with ISO/IEC 17025 and other relevant standards.
Thanks to automatic versioning, audit-proof archiving and individual workflows, you can organize the review and approval of new and revised work and process instructions efficiently and transparently. During an audit, you can provide auditors with an overview of all relevant QM documents including metadata (e.g. resubmission date, status, revision) with just a few clicks.
With roXtra, you can optimize quality management in the laboratory, minimize sources of error and ensure complete traceability - digitally, securely and in compliance with standards.
Digitally control and optimize processes in the laboratory
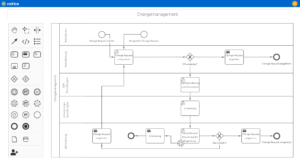
Optimize your quality management in the laboratory by digitizing and automating internal processes with roXtra Processes. With the help of graphical modeling (BPMN 2.0), you can define rules, responsibilities and tasks for laboratory processes - from test reports and corrective measures to sampling or dealing with complaints.
The Flowchart Designer enables quick visualization of flowcharts, organization charts and mind maps, so that workflows in the laboratory are presented in a structured and comprehensible way.
The Flowchart Designer enables quick visualization of flowcharts, organization charts and mind maps, so that workflows in the laboratory are presented in a structured and comprehensible way.
With roXtra Processes , you can make your process management in the laboratory transparent, automated and audit-proof - for more quality, traceability and standard-compliant processes.
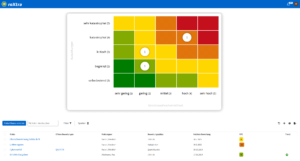
Structured risk management for laboratories
With roXtra Risks you can optimize your risk management in the laboratory, reduce your workload and ensure systematic risk analysis. Identify, evaluate and manage potential risks centrally in order to design your laboratory processes safely and in compliance with standards. The software supports common standards and regulations, including ISO 22367, ISO 15189, Rili-BÄK, ISO 31000, ONR 49001, ISO 9001 and ISO 27001.
Regular risk assessments in the laboratory are essential in order to detect and avoid hygiene deficiencies, equipment faults, incorrect calibrations or operating errors at an early stage. A structured risk management system enables the prevention, reduction and optimization of risks, improves compliance and ensures traceable documentation - a decisive factor for audits and certifications.
With roXtra Risks , you create a transparent, digital solution for your risk management in the laboratory - compliant, secure and future-proof.
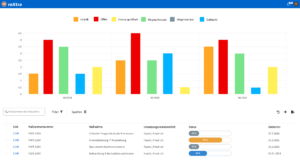
Professionally manage audits in the laboratory
Audits are a central component of quality management in the laboratory and require a structured, standard-compliant procedure. With roXtra Audits you can optimize your audit management and carry out internal and external audits in accordance with ISO 19011 efficiently and transparently.
The software enables you to access audit questionnaires from any location - even offline - and to manage audit processes digitally. This allows you to keep track of past, current and planned audits at all times and identify non-conformities and potential for improvement in a targeted manner. Immediate, corrective and preventive measures can be documented and implemented directly in the audit context.
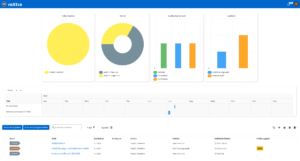
Action management for laboratories
Structured action management in the laboratory is essential to eliminate deviations, minimize risks and meet the requirements of relevant standards such as ISO 15189, ISO/IEC 17025 and GMP/GLP. With roXtra Actions you can manage immediate, corrective and preventive actions (CAPA) systematically, digitally and traceably.
The software enables seamless documentation and transparent monitoring of all measures to ensure compliance with regulatory requirements and continuous improvement.
Your advantages with roXtra Actions:
- Central management: Clearly record and control all measures.
- Automatic effectiveness check: Sustainable implementation through planned checks.
- Simple evaluation: Filter lists of measures and export them as Excel, Word or PDF.
- Cross-module: Linking for consistent documentation.
Support for quality management and certifications in laboratories
Our software solutions for laboratories support you in your daily laboratory operations as well as in the certification and re-certification of your quality management system. With roXtra, you can efficiently implement a wide range of standards and regulations and make your quality management in the laboratory safe, transparent and compliant.
Quality management standards
- ISO/IEC 17025 - Requirements for the competence of testing and calibration laboratories
- ISO 15189 - Quality management in medical laboratories (updated 2022)
- DIN EN ISO 9001 - Requirements for quality management systems
Risk management standards
- ISO 22367 - Risk management for error reduction in medical laboratories
- ISO 31000 - General guidelines on risk management
- ONR 49001 - Austrian standard for systematic risk management
Information security management
- ISO/IEC 27001 - Requirements for information security management systems (ISMS)
Specific guidelines and laws
- Rili-BÄK - Quality assurance of laboratory medical examinations
- KonTraG & Section 91 (2) AktG - Regulations on risk control in companies
roXtra supports you with the following solutions
We will show you roXtra in a free and non-binding online presentation.