GxP compliance with QM software from roXtra
Your quality management: compliant with EU GMP, GAMP 5, ISO 13485 and other guidelines
GxP Compliance with roXtra Software
Your quality management: compliant with EU GMP, GAMP 5, ISO 13485 and other guidelines
 |  |  |  |  |  |
 |  |  |
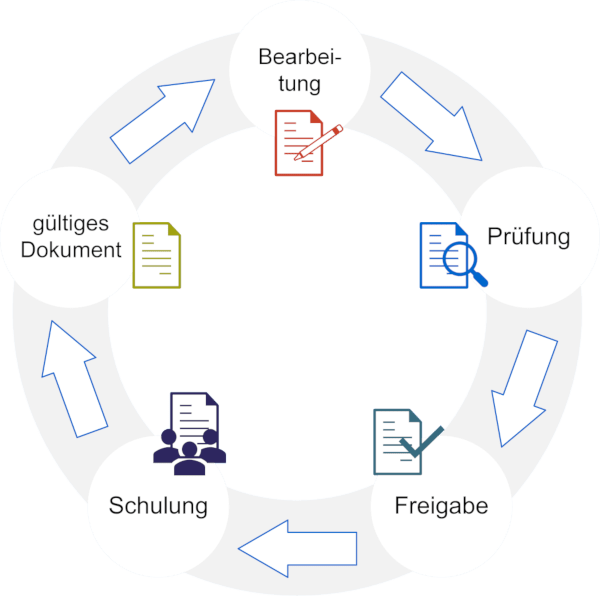
Proven functions for use in the GxP-regulated environment
Access and user management
roXtra enables role-based permissions assignment and ensures that only authorized users can access quality-relevant content. Access is documented and traceable.
- Role and rights-based access system
- Traceability of user activities
- Integration with Active Directory or SSO (optional)
Discover roXtra
Get to know our system in a non-binding and free online presentation.
Frequently asked questions about software validation
What does software validation mean in the GxP environment?
Software validation is the documented proof that a software system functions reliably and in compliance with regulations in the intended application environment – a central component of GxP compliance.
The term validation is also used in regulated industries for manufacturing processes, cleaning procedures or analyses – in software it refers to the compliant and traceable system functionality.
In short: Validation ensures that your software does exactly what it is supposed to do according to the specifications – reliably, reproducibly and GxP-compliant.
Why does software need to be validated?
Various regulations, such as the German Medical Devices Act (AMWHV), the Medical Devices Act (MPV), ISO 13485, and the EU GMP Guidelines, require the validation of software in regulated areas. This requirement serves to minimize risk, protect patients, and ensure complete traceability.
Software validation demonstrates that computer-based systems function safely, reliably and according to their specifications – a key aspect of GxP compliance.
Simply put, software is a critical tool. Only regular validation can ensure its correct use – comparable to the maintenance of technical equipment.
Which software needs to be validated?
Not all software in a company is subject to validation requirements. According to GxP regulations, only those systems that directly impact product-relevant processes or the provision of regulated services must be validated.
Software validation follows a risk-based approach: Systems such as email or operating systems generally do not require validation – in contrast to quality-critical software, such as those used to control manufacturing processes.
Who is responsible for software validation?
According to GxP regulations, the responsibility for software validation lies with the user—that is, the company that uses the software in a regulated environment. They are obligated to demonstrate that the system is suitable for its intended purpose.
However, the manufacturer can provide support with validation – for example through sample documentation, validation aids or advice.
For more information, see the section: “Does Roxtra GmbH support me with software validation?”
When should software be validated – and when should it be revalidated?
Software validation must be performed before first productive use – this is required by regulations such as DIN EN ISO 13485:2016 (Chapter 4.1.6). Revalidation is necessary for changes to the system. However, not every change requires full validation. The key is the risk assessment:
- Major updates (e.g. roXtra 8 → 9) usually bring profound changes and require a complete revalidation.
- Minor updates (e.g. 9.20.0 → 9.22.0) can possibly be adequately covered with a targeted partial revalidation.
Changes in the area of application – such as rollout to new business areas – can also trigger revalidation.
Tip: The criteria and processes for future revalidations should be defined during the initial validation. The release notes in the system (e.g., under Help > General > Release Notes in roXtra) provide guidance.
Does Roxtra GmbH support me with software validation?
Yes – with roXtra, you rely on a proven, GxP-compliant QM software that has been successfully used by numerous customers in highly regulated industries – from pharmaceutical companies to medical device manufacturers – for many years. As a software provider, we ensure that roXtra is extensively tested before delivery and is available to you as a validation-friendly standard solution. This allows you to significantly reduce the validation effort within your company.
We also provide you with practical sample documentation that illustrates the validation process – from the validation plan to the validation report. This provides you with a structured basis for your own documentation. With Roxtra, you have a reliable and experienced partner at your side who understands the requirements of the GxP-regulated environment – and supports you in implementing your software validation efficiently and securely.
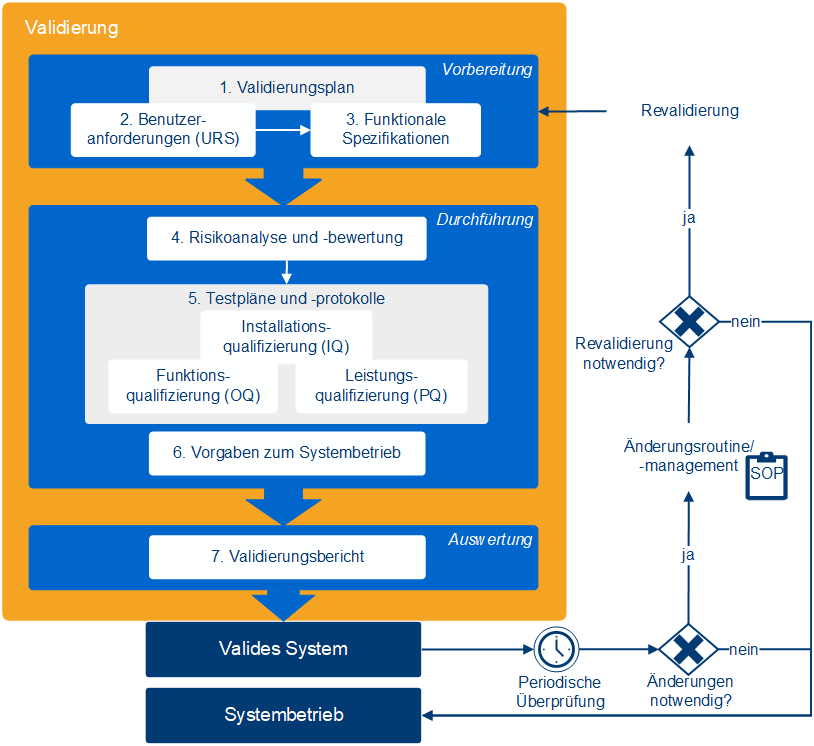
What does software validation involve?
Software validation consists of several documented steps that demonstrate the suitability of a system for its intended use in a GxP-regulated environment. This includes not only testing but also requirements, risks, responsibilities, and operational specifications. The goal is to comprehensively ensure the system's functionality, traceability, and compliance.
This includes:
- Validation report: Documentation of results and formal release
- Validation planning: definition of purpose, scope and responsibilities
- Requirement & Specification: Matching user requirements and system functions
- Risk assessment: Analysis of potential risks and appropriate countermeasures (e.g., testing, training)
- Operating specifications: revalidation criteria, backup regulations, emergency measures
Further information on GxP compliance with roXtra
The guidelines for good working practice (GxP)
Why roXtra GxP?
For companies in regulated industries such as medicine, pharmaceuticals, or pharmaceutical chemistry, the use of compliant and tested systems is essential. With roXtra GxP, you receive a GxP-compliant standard solution that meets strict national and international requirements – while significantly reducing qualification and validation effort.
Whether you are setting up, maintaining or further developing your QM system: roXtra GxP and the experienced team at Roxtra GmbH will support you in implementing efficient, compliant and future-proof quality management.
What are GxP guidelines for – and what does this mean for your software?
Good Manufacturing Practice (GxP) guidelines place high demands on processes, products, and the software used. The goal is always the safety of patients, users, and consumers. Whether GMP (Good Manufacturing Practice), GLP (Good Laboratory Practice), or GDP (Good Distribution Practice), all areas require consistent monitoring and documentation of product-relevant influences.
Our software solutions support you in complying with legal requirements such as the AMG, MPG, 21 CFR Part 11, as well as in the implementation of standards such as ISO 9001, DIN EN ISO 13485 and regulatory standards such as the EU GMP Guidelines (Annex 11, 15), GAMP 5, FDA guidelines, CE marking and Rili-BÄK.
👉 Learn here how specific GxP requirements are implemented with roXtra.
What does GxP compliance mean?
GxP compliance refers to adherence to industry-specific quality and safety standards in regulated areas such as pharmaceuticals, medical technology, and biotechnology. GxP encompasses guidelines such as GMP (manufacturing), GLP (laboratory), and GCP (clinical trials).
The aim is to ensure the quality of products and the integrity of data through documented, traceable processes – often with the help of GxP-compliant QM software.
What are GxP guidelines – and what does GxP compliance mean?
GxP guidelines are regulatory requirements that ensure that products are effective, safe, and of assured quality in highly regulated industries such as pharmaceuticals and medical technology. "GxP" stands for various "good practice" standards – e.g., GMP, GLP, GCP, or GVP.
GxP conformity (also known as GxP compliance) means that companies adhere to these standards through documented, traceable, and auditable processes. It is crucial for approvals, audits, and the legally compliant use of QM software in regulated environments.
What does GxP mean in pharmacy?
GxP in pharmacy encompasses all legal quality guidelines that must be adhered to during the development, manufacture, testing, and storage of medicinal products – e.g., GMP (Good Manufacturing Practice) or GCP (Good Clinical Practice).
These requirements ensure product safety, efficacy, and traceability and are essential for approvals, inspections, and GxP compliance in the pharmaceutical environment. Especially in the GxP pharmaceutical industry, they form the basis for regulatory-compliant processes and sustainable quality management.
We will show you roXtra in a free and non-binding online presentation.