The QM solution for pharmaceutical manufacturers
The QM solution for pharmaceutical manufacturers
Success Stories
Find out first-hand how pharmaceutical manufacturers use roXtra to make their pharmaceutical quality management efficient and compliant. Whether GMP guidelines, ISO 9001 or ISO 13485 - roXtra offers specially developed solutions for quality management in the pharmaceutical industry.
In our blog, you can find out more about certification and re-certification, the implementation of pharmaceutical quality management in practice and practical application examples from the industry. Discover how our software can help you to reliably meet regulatory requirements and optimize processes.
Get advice now
Get to know roXtra in a non-binding and free online presentation.
Action management in the pharmaceutical industry
Effective action management is a central component of quality management in the pharmaceutical industry in order to identify deviations at an early stage, minimize risks in a targeted manner and reliably meet legal requirements - for example in accordance with ISO 9001 or GMP. With roXtra Actions immediate, corrective and preventive actions (CAPA) can be systematically planned, controlled and fully documented.
Roxtra's software ensures transparent tracking of all measures - even in a GxP/GMP-regulated environment - and thus supports the sustainable optimization of your processes. This not only strengthens your pharmaceutical quality management, but also ensures compliance with all relevant regulatory requirements.
Your advantages with roXtra Actions:
- Central management: Clearly record and control all measures.
- Automatic effectiveness check: Sustainable implementation through planned checks.
- Simple evaluation: Filter lists of measures and export them as Excel, Word or PDF.
- Cross-module: Linking for consistent documentation.
One software, many application areas.
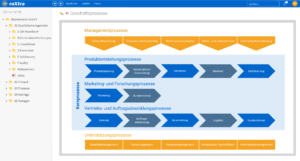
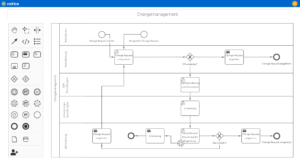
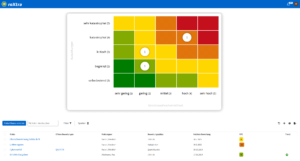
What is pharmaceutical quality management?
Pharmaceutical quality management refers to all systematic measures aimed at ensuring the quality, safety and efficacy of medicinal products throughout the entire manufacturing and distribution chain. It includes processes such as document control, risk management, audits and CAPA as well as compliance with regulatory requirements such as GMP (Good Manufacturing Practice).
Why is quality management so important in the pharmaceutical sector?
Quality management is essential to guarantee patient safety, meet regulatory requirements and ensure consistently high product quality. In the pharmaceutical industry, errors can have serious consequences - which is why structured QM systems and seamless documentation are essential.
What role does GxP or GMP software play in pharmaceutical quality management?
GMP software supports pharmaceutical manufacturers in complying with legal regulations and standards through digital, traceable processes. It helps to manage QM documents, processes, audits, risks and measures in a structured manner and to document them in an audit-proof manner - a central building block for efficient and compliant quality management in the pharmaceutical industry.
roXtra supports you with the following solutions
We will show you roXtra in a free and non-binding online presentation.








