Your solution for quality management in medicine
Your solution for quality management in medicine
Already in use in over two thirds of German university hospitals
: our software solutions









Success Stories
Find out first-hand how university hospitals use roXtra to make quality management in medicine efficient and compliant. Whether ISO 9001, ISO 7101, KTQ or comprehensive risk management - roXtra offers customized solutions for structured and standard-compliant medical quality management that are specially tailored to the requirements of hospital operations.
In our blog, you can find out more about certification and re-certification, the implementation of quality management in medicine and practical application examples from the industry.
Get advice now
Get to know roXtra in a non-binding and free online presentation.
Why does medicine need quality management?
Quality management in medicine is essential to ensure safe, efficient and patient-oriented care. Structured medical quality management helps university hospitals to comply with legal requirements and medical standards, optimize processes and minimize risks.
In addition, efficient quality management helps to increase patient safety, drive continuous improvements and overcome economic and organizational challenges in modern medicine.
What does quality management do for medicine?
Effective and structured quality management in medicine ensures transparent processes in university hospitals. It includes compliance with legal requirements, the optimization of clinical processes and the continuous improvement of various standards. Effective medical quality management minimizes risks, increases patient safety and supports specialists in carrying out their work efficiently and in compliance with regulations.
What quality management regulations must university hospitals comply with?
University hospitals in Germany are subject to various quality management regulations. Central to this are the provisions of the German Social Code (SGB V, § 135a and § 137), which prescribe continuous quality assurance and reporting. The Federal Joint Committee (G-BA) also stipulates specific guidelines, including the guideline on cross-institutional quality assurance (Qesü-RL). Many clinics follow the international standard DIN EN ISO 9001 or are certified according to the KTQ procedure (Cooperation for Transparency and Quality in Healthcare).
In addition, university hospitals must comply with EU regulations such as the Medical Device Regulation (MDR), particularly when dealing with medical devices and clinical research. In addition, occupational health and safety and hygiene regulations, including the Infection Protection Act (IfSG) and the recommendations of the Robert Koch Institute (RKI), govern key aspects of patient safety. Many hospitals are also developing internal quality management systems to ensure the highest standards of patient care.
How does QM in a university hospital differ from that in a normal hospital?
Quality management (QM) in a university hospital differs from that in a normal hospital primarily due to the additional requirements for research and teaching. In addition to patient safety and medical care, which play a central role in both types of hospital, university hospitals must also meet high quality standards in clinical research and the training of medical students and specialist staff. These include compliance with Good Clinical Practice (GCP) in clinical trials, the Medical Device Regulation (MDR) for the safe use of medical devices and strict ethical and scientific standards.
In addition, university hospitals often work together with several external partners, such as research institutes and universities, which requires a particularly complex QM system. Furthermore, they are often certified according to specialized standards such as DIN EN 15224 or ISO 9001 and must meet the requirements of funding bodies and scientific societies in addition to legal requirements. Overall, QM in university hospitals is therefore more comprehensive, more multidisciplinary and more focused on innovation and knowledge transfer.
To what extent does QM influence the further training and qualification of medical staff?
Quality management (QM) ensures the further training and qualification of medical staff in university hospitals through fixed standards, regular training and certifications such as ISO 9001 or KTQ. Digital training platforms, on which employees can flexibly access training content, as well as standard operating procedures (SOPs) and simulation training ensure practical training. In addition, professional associations and state medical associations require certified further training. In this way, QM contributes to continuous improvement and safe patient care.
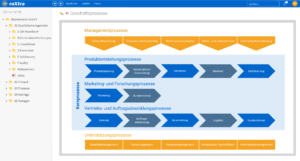
One software, many application areas.
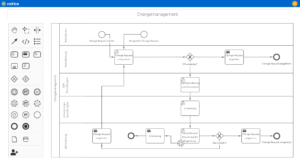
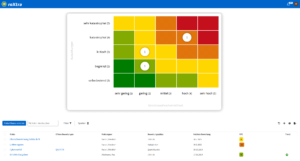
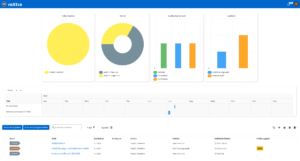
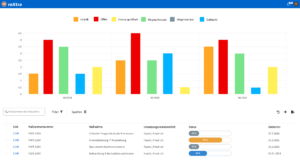
SaaS or on-premise
As a customer, you decide whether you want to use the roXtra QM software as Software-as-a-Service (SaaS) or hosted locally (on-premise). Please note that the roXtra software is developed and hosted in Germany.
Regardless of which model you choose, security is our top priority: with regular penetration tests (pentests) and security checks, we guarantee the protection of your data and aim to meet the highest security standards.
Is hosting your SaaS solution in accordance with the C5 standard of the German Federal Office for Information Security (BSI) relevant for you? Contact us to find out more.


roXtra supports you with the following solutions
We will show you roXtra in a free and non-binding online presentation.